Surfactants, also known as surface active agents or interfacial active agents, are compounds that significantly reduce surface tension or interfacial tension between two liquids, liquid-gas, or liquid-solid phases. In everyday life, various detergents such as soap, facial cleansers, etc., contain surfactants as active ingredients. In biochemical experiments, surfactants are required for cell lysis. So, what is the principle behind their action?
Surfactants generally consist of two parts: Hydrophilic moiety - composed of one or more polar groups (hydrophilic base); Lipophilic moiety - composed of nonpolar hydrocarbon chains (lipophilic base).
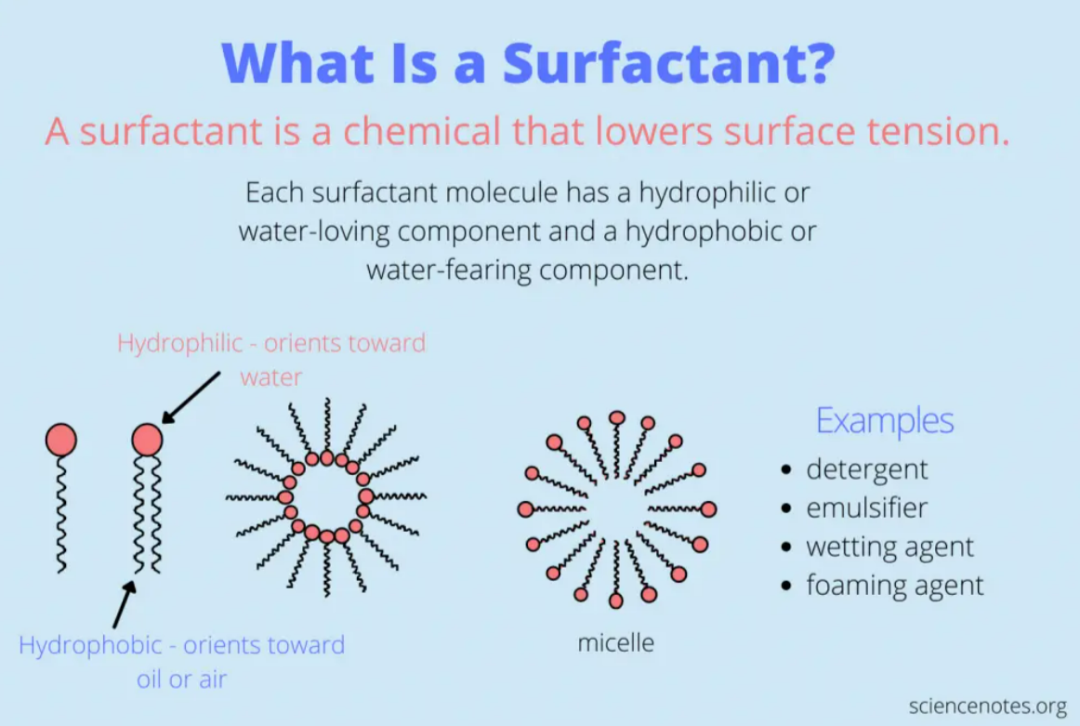
Surfactant Structure Schematic Diagram (Image Source: sciencenotes.org)
Based on the molecular structure and dissociation properties of polar groups, surfactants can be categorized into anionic surfactants, cationic surfactants, amphoteric surfactants, and nonionic surfactants.
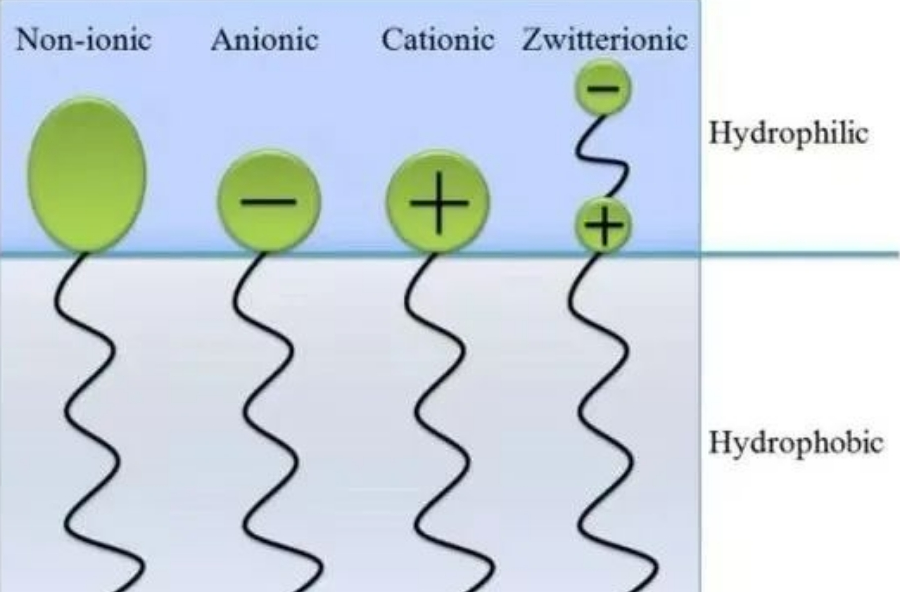
Structures of Four Types of Surfactants
3.1 Ionic Surfactants
Ionic surfactants are surfactants with a charged hydrophilic end. They can be further classified into three types based on the nature of the charge:
Cationic Surfactants Cationic surfactants are mainly divided into ammonium salt type and quaternary ammonium salt type, primarily used for sterilization and disinfection. Example: Benzalkonium chloride (BZK), Cetrimonium chloride.
Anionic Surfactants Anionic surfactants typically contain sulfate, sulfonate, or carboxylate groups. Example: Sodium dodecyl sulfate (SDS).
Zwitterionic/Amphoteric/Ampholytic Surfactants These surfactants have both cationic and anionic groups on the hydrophilic end, resulting in a net charge of zero. They can react with both acids and bases, exhibiting a cationic nature in acidic solutions and an anionic nature in alkaline solutions. They can be further categorized into betaines, amino acid derivatives, imidazoline derivatives, and amine oxides.
Nonionic Surfactants The hydrophilic end of nonionic surfactants is uncharged. Their hydrophilic-lipophilic balance (HLB) measures the balance between hydrophilicity and lipophilicity, with higher HLB values indicating greater lipophilicity and vice versa. Generally, nonionic surfactants are considered more compatible and do not cause protein denaturation. Examples: Polyethylene glycol octylphenyl ether (Triton X-100), Tweens, polyoxyethylene types (Myrij, Brij, Perogol O, Emolphor, etc.).
Surfactants can reduce the surface tension of liquids, causing surface molecules to have an inward tendency and exerting a force that automatically contracts the surface to the minimum area. For example, oil and water are immiscible, but when surfactants are present, they reduce the surface tension at the interface of oil and water, thereby promoting their mutual solubility.
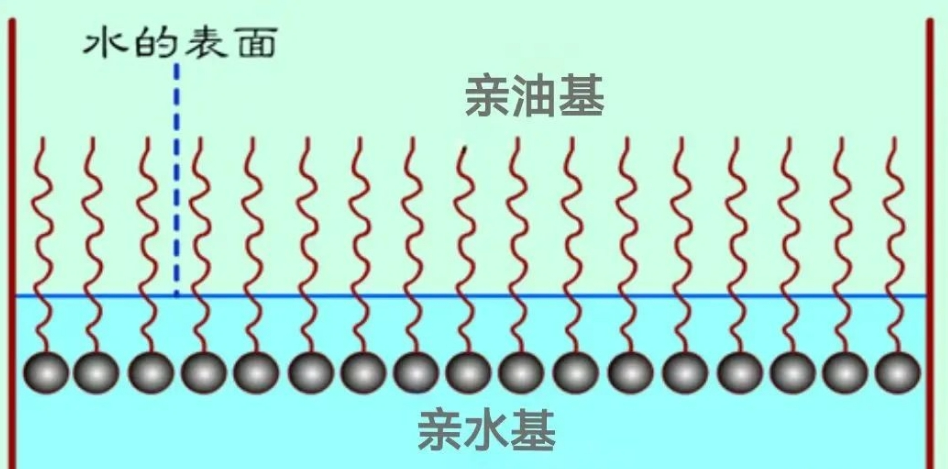
Orientational Arrangement of Surfactants in Water
In biochemical experiments, surfactants are required for cell lysis. So, what is the principle behind their action?
To answer this question, let's begin with the biological structure of the cell membrane. The cell membrane is composed of a phospholipid bilayer (Figure a). In the quiescent state, the cell membrane on the cytoplasmic side is primarily rich in anionic and amine-containing phospholipids, such as phosphatidylserine (PS) and phosphatidylethanolamine. On the outer side, the cell membrane is mainly rich in choline-containing phospholipids, such as sphingomyelin and phosphatidylcholine. When the cell is activated or undergoing apoptosis, negatively charged phospholipids flip outward (this is also the principle behind Annexin V detecting cell apoptosis, as Annexin V has high affinity for PS).
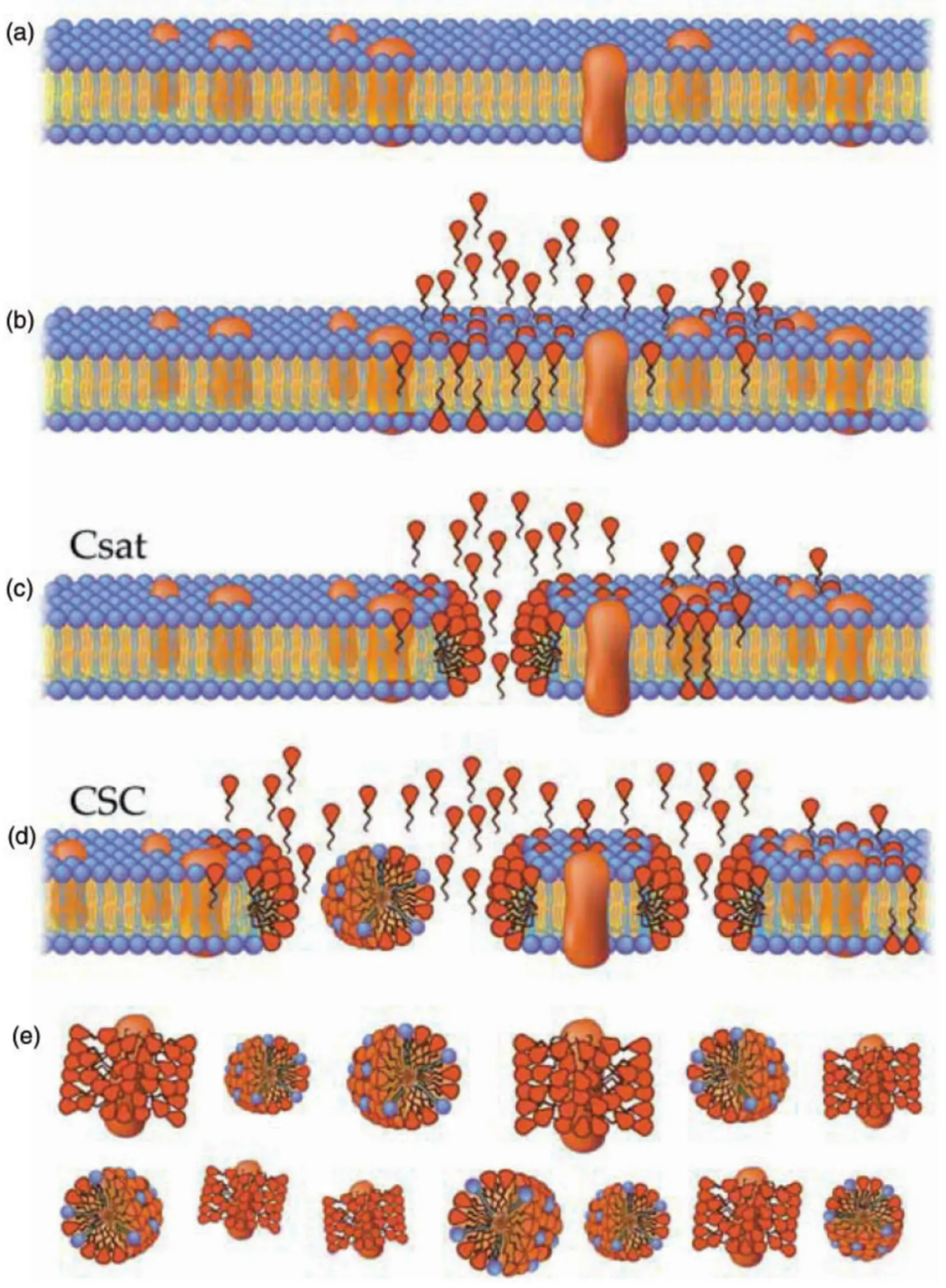
Interaction between Surfactants and Cell Membrane (Image Source: Parsi et al., 2016)
When the concentration of surfactants is low, surfactant monomers integrate into the phospholipid bilayer without disrupting its structure (Figure b). However, when the surfactant concentration exceeds the critical saturation concentration (Csat), monomers aggregate within the cell membrane, leading to its structural disruption (Figure c). Upon reaching the critical solubilization concentration (CSC), where the surfactant-to-lipid ratio is critical, the phospholipid bilayer dissolves, forming surfactant-lipid micelles and short membrane fragments (Figure d). As the dissolution progresses, only surfactant-lipid micelles and membrane fragments enveloped by surfactants remain. Surfactants also replace lipids, interacting with the hydrophobic ends of membrane proteins (Figure e).
5.1 Critical Micelle Concentration
Surfactant molecules consist of two parts, ideally with the hydrophilic portion in water and the hydrophobic portion outside water. When surfactants are added to water, they initially aggregate at the surface, with their tails extending into the water. Once a certain concentration of surfactants is reached, their tails can no longer extend out of the water, and some of them are forced to sink beneath the surface, entering the solution. Upon adding more surfactants, the molecules transition into the solution. Due to the presence of hydrophobic groups, the repulsive forces between water molecules and surfactant molecules outweigh the attractive forces, leading to the formation of micelles with hydrophobic groups oriented inward and hydrophilic groups outward, stably dispersed in water. Within a certain temperature and concentration range, surfactant micelles have a specific degree of molecular association, and different surfactants exhibit varying molecular association numbers in their micelles. The minimum concentration at which surfactant molecules aggregate to form micelles is known as the Critical Micelle Concentration (CMC). The CMC varies among different surfactants, and for surfactants within the same series with identical hydrophilic groups, a larger hydrophobic group corresponds to a smaller CMC.
5.2 Hydrophilic-Lipophilic Balance (HLB) Value
The selection of surfactants is based on the Hydrophilic-Lipophilic Balance (HLB) value, which represents the combined affinity of the hydrophilic and lipophilic groups in surfactant molecules for oil or water.
HLB is a relative value, with the HLB value for completely hydrophilic wax set at 0, and the HLB values for surfactants ranging from 0 to 40. For nonionic surfactants, the HLB range is 0 to 20, with higher HLB values (HLB > 9) indicating hydrophilic surfactants and lower values (HLB < 9) indicating lipophilic surfactants.
The HLB value of a surfactant is closely related to its application. Surfactants with HLB values in the range of 3 to 6 are suitable as water-in-oil (W/O) emulsifiers. Those with HLB values in the range of 7 to 9 are suitable as wetting agents and penetrants. Surfactants with HLB values in the range of 8 to 18 are suitable as oil-in-water (O/W) emulsifiers, while those with HLB values in the range of 13 to 18 are suitable as solubilizers and dispersants.
6.1 Solubilizing Agent
When the concentration of surfactants in an aqueous solution reaches the Critical Micelle Concentration (CMC), micelles form. This enables the complete dissolution of substances that are otherwise insoluble or sparingly soluble in the solution, resulting in a thermodynamically stable solution, thereby acting as a solubilizing agent.
6.2 Emulsifier
Surfactants can reduce the interfacial tension between two immiscible liquids, such as oil and water, allowing one phase to disperse as droplets in the other, forming a stable emulsion. The oil-water dispersion systems formed through emulsification include water-in-oil (O/W) and oil-in-water (W/O) emulsions.
6.3 Wetting and Penetrating Agent
Wetting refers to the process where gas on the surface of a solid is replaced by water. Surfactants can significantly reduce the surface tension of water, allowing water to rapidly spread on the solid surface, facilitating the wetting process. Wetting and penetrating actions are essentially similar, with the former acting on the interior of the solid, while the latter acts on the external surface.
6.4 Dispersant
The system formed by evenly dispersing insoluble solid particles into a liquid is called a dispersion or suspension. The surfactant that enables this dispersion is known as a dispersant. For a surfactant to be an effective dispersant, it must have three functions. Firstly, it must exhibit good wetting properties to ensure thorough wetting of each solid particle, replacing air in the particles, and further causing the particles to fracture into smaller crystals. Secondly, it must significantly reduce the interfacial tension between solid and liquid, increasing the adsorption and compatibility between solid and liquid, thereby reducing the system's internal energy. Lastly, it must form a mechanically strong interfacial membrane around solid particles in the form of a hydrated layer or charged layer, preventing the aggregation of solid particles.
6.5 Foaming Agent
Bubbles constitute a thermodynamically unstable system prone to rupture due to the drainage of liquid film layers between bubbles and the coalescence of small bubbles into larger ones. If surfactants are present in the liquid, surfactant molecules adsorb onto the bubble surface and arrange themselves, reaching a certain point where the bubble wall becomes a sturdy, thin film. This makes it difficult for bubbles to coalesce. Simultaneously, the oriented arrangement of surfactants on the liquid surface reduces the surface tension of the liquid, leading to a decrease in the pressure difference between bubbles, slowing down the drainage rate. These two effects reduce the ability of bubbles to rupture, facilitating bubble formation and stable existence.
6.6 Detergent
The process of removing foreign substances or dirt from the surface of a solid immersed in a medium (often water) is known as washing, and chemicals that can perform washing actions are called detergents, with surfactants commonly serving as the main component. The washing action is a complex process resulting from the combined effects of surfactants, including wetting, emulsification, dispersion, and solubilization, along with mechanical actions such as stirring, rubbing, and the flow of water.