Surfactants are a class of structurally unique organic compounds with a long history and diverse types [1,2]. In the traditional molecular structure of surfactants, both hydrophilic and hydrophobic parts are present, giving them the ability to reduce water surface tension—hence the origin of their name. In the classification of disciplines, surfactants fall within the realm of colloid and interface chemistry under physical chemistry, while also having intricate connections with other disciplines. For instance, surfactants can spontaneously form highly ordered supramolecular structures in solutions, contrary to the increase in entropy law in thermodynamics. Various self-assembled structures fall within the scope of nanoscience and can serve as templates for synthesizing other nanomaterials. The vesicles in self-assembled structures, resembling the structure of cell membranes, can be used as carriers for drug delivery, and so on. These characteristics make surfactant research ever-expanding and continually innovative; the development of chemical industry and improvements in organic synthesis processes continuously drive the innovation of surfactants. Therefore, it can be said that the science of surfactants is both ancient and youthful, and to this day, it continues to bring us surprises.

People's understanding of surfactants mostly originates from various cleaning products. Indeed, cleaning is the oldest and most widely known function of surfactants in history, and it remains well-known to this day. However, the story of surfactants is much longer than we might imagine, even closely related to the activities and origins of life. Surfactants have been intertwined with us since the beginning of life, naturally existing in the human body and the bodies of animals and plants, including the skeletal framework of cell membranes, pulmonary surfactants, and bile acids that play a crucial role in fat metabolism. In recent years, as the theory of life originating from deep-sea hydrothermal vents has gained widespread support, the crucial role of surfactants in the origin of life is being recognized. More and more people believe that phospholipids provided the essential conditions for the initial emergence of life, breaking free from the rock pores. Subsequently, other members such as surfactant proteins and bile salts joined the regulatory pathways of life, constructing a diverse and vibrant world.
The early surfactants used by humans, mainly soaps and detergents, also typically came from living organisms. Approximately 5000 years ago, ancient Egyptians discovered that sheep tallow carbon ash could be used as a detergent. In ancient China, soapnuts and "zao dou" (commonly known as soapberries) and Europe's Turkish red oil are classic examples of ancient human use of surfactants. With the development of the times, these natural surfactants were no longer suitable for the needs of industrial and agricultural production and daily life. Thanks to the development of synthetic chemistry and advances in chemical processes, a variety of surfactants have been developed, and their structures have undergone significant changes, accompanied by the development of new concepts. Below, we will provide a brief overview of the field and its trends from three aspects: the classification of surfactants, their basic properties, and new developments.
Surfactants can be classified from different perspectives such as structure, function, and source. In terms of structural classification, they can be categorized based on the types of hydrophilic and hydrophobic groups and the overall molecular configuration. In conjunction with Figure 1, we will introduce the basic structure of surfactants, the common head groups, and types of hydrophobic tail chains, along with representative special structures.
2.1 Classification Based on Hydrophilic Groups
For a long time, the classification of surfactants mainly relied on the types of hydrophobic groups. According to this method, surfactants can be roughly divided into four categories: anionic surfactants, cationic surfactants, amphoteric surfactants, and nonionic surfactants.
Anionic surfactants (Figure 1(a)) dissociate in water, causing the head group to carry a negative charge. This is the oldest type of surfactant, including bile acids, soap-based surfactants, and some phospholipids. In addition to the carboxylate ions in bile acids and soap-based surfactants and the phosphate ions in phospholipids, sulfate and sulfonate ions were later developed, further enriching the types of anionic surfactants. In contrast, cationic surfactants (Figure 1(b)) have a positively charged head group after dissociation, with quaternary ammonium salts being the most common. Aqueous solutions of cationic surfactants have strong bactericidal properties and are often used for disinfection. Since many solid surfaces, such as glass, carry a negative charge, cationic surfactants are more easily adsorbed, making the surface hydrophobic. Cationic surfactants with two tail chains can provide fabric softening and antistatic properties in the textile industry, giving them irreplaceable advantages. Both anionic and cationic surfactants are sensitive to temperature. Lowering the temperature reduces the ability of surfactant molecules to dissociate polar groups and the flexibility of hydrophobic tail chains, leading to a decrease in efficiency. When the temperature reaches a critical point, surfactants crystallize and precipitate, causing a sharp decrease in surface activity; this temperature is referred to as the Krafft point.
Amphiphilic surfactants are formed when two head groups with opposite charges are covalently connected (Figure 1(c)). Natural amphiphilic surfactants, such as lecithin, contain both phosphate ions and quaternary ammonium cations, playing essential roles in regulating life activities and serving as important additives in the food industry. For artificially synthesized amphiphilic surfactants, betaine, which contains both quaternary ammonium cations and carboxylate ions, is known. These surfactants are not only diverse but also industrially produced on a large scale. Additionally, some amine oxide surfactants exhibit amphiphilic properties due to the uneven distribution of their charges between nitrogen and oxygen.
In contrast to ionic surfactants, nonionic surfactants (Figure 1(d)) have a head group that hardly dissociates in water, and their water solubility primarily relies on hydrogen bonding between the head group and water molecules. Common head group structures include oligomeric ethylene oxide, oligosaccharides, etc. In terms of molecular size, nonionic surfactants include small molecules like CnEm and alkyl glucosides, as well as large-sized poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers (commercially known as Pluronic). Due to the absence of electrostatic repulsion between head groups, nonionic surfactants arrange more closely at the air-water interface, enhancing their ability to reduce water surface tension. Nonionic surfactants exhibit high stability and are less affected by pH, heavy metal ions, and electrolytes, showing excellent tolerance. In contrast to ionic surfactants, nonionic surfactants have good low-temperature resistance, but their surface activity is lost at high temperatures due to the destruction of hydrogen bonding structures, resulting in the overall sample appearing turbid. The temperature at which this transition occurs is called the cloud point. To ensure the effectiveness of surfactants, the usage temperature for ionic surfactants must be higher than their Krafft point, while for nonionic surfactants, it must be lower than their cloud point.
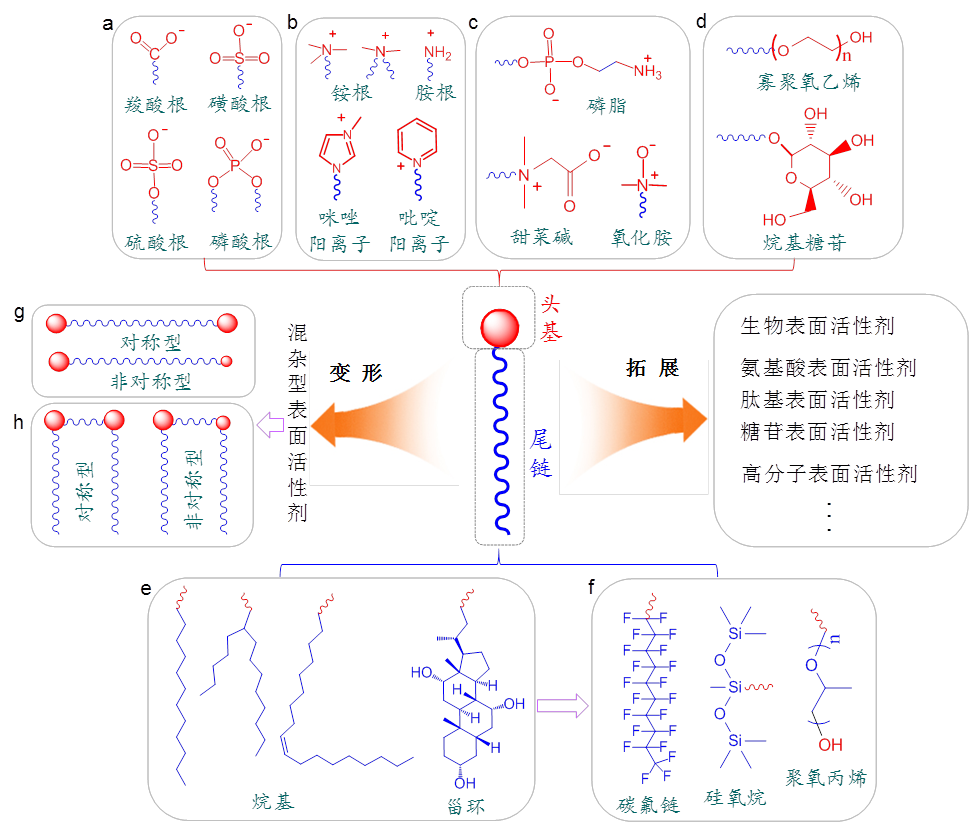
Figure 1: Basic structures of surfactants, common head groups, and types of hydrophobic tails, as well as some special categories of surfactants. (a)~(d) Main types of surfactant head groups: anionic, cationic, amphoteric, and nonionic. (g), (h) Two special structures of surfactants, namely, Bola-type and Gemini-type. (e), (f) Common structures of surfactant hydrophobic tails.
2.2Division based on hydrophobic tail
As mentioned earlier, for a long time, the types of hydrophobic tails in surfactants were relatively limited. For the most common alkyl, in addition to straight-chain types, there are also branched alkyls (such as sodium bis(2-ethylhexyl)sulfosuccinate, AOT) and unsaturated alkyls (such as sodium oleate). Additionally, there are cyclic and rigid cholestane frameworks (Figure 1(e)). With the continuous expansion of surfactant types, the variety of tail types has also gradually increased. Among them, perfluorinated surfactants are formed by replacing hydrogen atoms connected to carbon atoms on the hydrocarbon chain with fluorine atoms. In addition to having low surface tension, fluorocarbons are generally more stable than their corresponding hydrocarbon counterparts. The adsorption, aggregation, wetting, and adhesion properties of perfluorinated surfactants differ significantly from hydrocarbon surfactants [8]. Compared to hydrocarbon chains, perfluorinated surfactants are characterized by "three highs" – high surface activity, high thermal stability, and high chemical stability – as well as "two repellencies" – being both hydrophobic and oleophobic. Based on these characteristics, perfluorinated surfactants are widely used in the preparation of hydrophobic materials. Tadros considered perfluorinated surfactants to be more powerful wetting agents than hydrocarbon surfactants. He prepared a mixture of a perfluorinated surfactant and a hydrocarbon surfactant solution, which could simultaneously reduce the interfacial tension between water-hydrocarbons and water-fluorocarbons. Organosilicon surfactants have hydrophobic chains consisting of alternating silicon-oxygen bonds and methyl groups attached to silicon atoms. By finely controlling the hydrophobic groups, the performance of organosilicon surfactants can be altered . Due to the longer silicon-oxygen bond length and larger bond angle, the silicon-oxygen chain is more flexible compared to hydrocarbon chains. Organosilicon surfactants use methylated siloxane groups as hydrophobic groups and graft one or more hydrophilic groups, allowing them to exhibit good surface activity in both aqueous and non-aqueous solutions . Additionally, due to the inclusion of more terminal methyl groups, organosilicon surfactants also have high surface activity and strong hydrophobicity. Moreover, they have advantages such as corrosion resistance, high temperature resistance, and non-toxicity, making them irreplaceable in many fields. In polymer-type surfactants, polypropylene oxide (PPO) obtained by ring-opening polymerization of propylene oxide is often used as the hydrophobic chain. The structures of the above three hydrophobic segments are shown in Figure 1(f).
2.3Division based on molecular configuration
The discussion above is limited to cases where the hydrophilic/hydrophobic portions of surfactants have a single structure. With the continuous updating of surfactant structures, both hydrophilic head groups and hydrophobic tails have diversified combinations. Regarding hydrophilic head groups, surfactants with composite hydrophilic head groups, such as fatty alcohol polyethylene glycol (3) sulfonated succinate disodium salt (MES) containing both polyethylene glycol, sulfonate, and carboxylate groups; and alcohol ether phosphate ester (AEP) containing both polyethylene glycol (AEO) and phosphate groups, have been developed. These surfactants with composite hydrophilic head groups demonstrate better performance in areas such as infant care formulations and temperature- and salt-resistant additives than single-type surfactants. Regarding hydrophobic tails, the combination of alkyl and benzene rings has created widely used surfactants such as sodium dodecylbenzenesulfonate and alkylphenol polyoxyethylene ether. Combining alkyl and perfluorocarbon chains has created mixed hydrocarbon-perfluorocarbon surfactants, etc. [12]. Specifically, when two head groups are located at both ends of the hydrophobic tail, it is called a Bola-type surfactant (Figure 1(g)) [13]. Its surface activity depends on the length of the hydrocarbon chain and the nature of the head group. Generally, amphiphilic compounds with long alkyl chains have stronger surface activity. The Riviere research group [14] first synthesized symmetric Bola amphiphilic molecules with two amino acid head groups and various hydrophobic spacers. They observed the micellization of Bola amphiphilic reagents with a 20-carbon alkyl chain. Bola-type surfactants have a certain influence on the formation and stability of microemulsions and reverse microemulsions. Zhang et al. [15] found that changing the oil-water ratio can form two types of microemulsions that transform into each other. Under the emulsifying action of a small amount of Bola surfactant, O/W microemulsions exhibit good resistance to inorganic salts and temperature. Surface tension and interfacial tension reduction abilities of Gemini surfactants are 10 to 100 times higher than conventional surfactants. Moreover, some morphologies of solutions of Gemini surfactants show significant micelle shapes. Due to these characteristics, Gemini surfactants have found extensive applications [16]. In recent years, many studies have explored the influence of Gemini surfactant structure on its properties. Gemini surfactants' high surface activity and adjustable structure give them many advantages over single-chain surfactants. Since cationic Gemini surfactants can form complexes with various therapeutic macromolecules (gene drugs, vaccines, proteins, and peptides) and anticancer drugs [17], they are an excellent choice for designing gene carriers and drug delivery systems. Gemini surfactants have been further extended to form oligomeric surfactants with multiple heads and tails [18], and their performance is greatly influenced by the degree of oligomerization, spacer length, and hydrophobic tail length and shape. Whether Bola or Gemini, the contained head groups can be the same (symmetric) or different (asymmetric). For asymmetric surfactants, their non-uniform structure often brings unexpected performance.
2.4Other classification criteria
In addition to the above criteria, some surfactants are named based on their sources, such as biosurfactants, which refers to surfactants directly extracted from animals or plants or obtained through biotechnological processes such as fermentation. Some surfactants are named after a certain class of organic compounds due to their hydrophilic groups, such as amino acid surfactants, alkyl glycosides, and peptide surfactants. These surfactants are favored by people for their high biocompatibility in life sciences, daily chemical, washing, and skincare. Additionally, there are other classification criteria, such as dividing surfactants into small molecule surfactants and polymer surfactants based on molecular weight, and so on